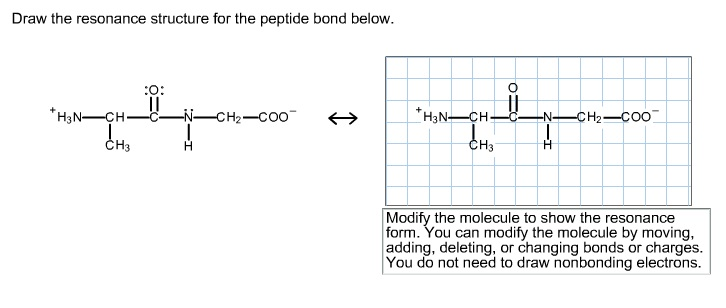
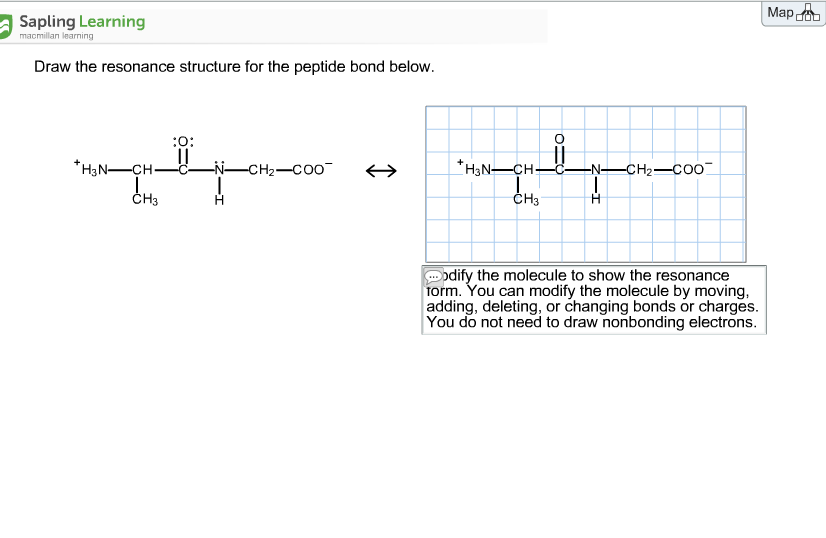
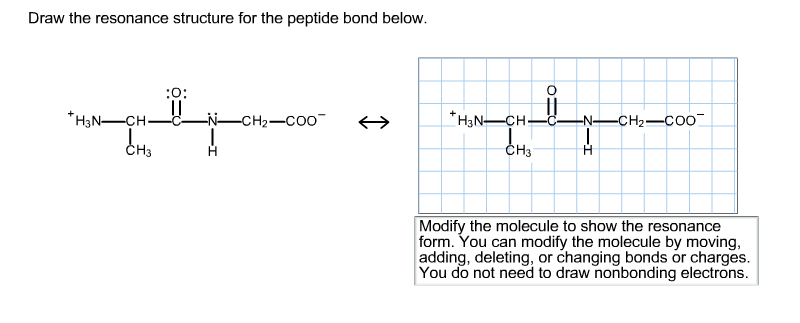
Select Draw Rings Groups More Erase. Modify the molecule by moving adding deleting or changing bonds or charges to show the resonance form.

Solved Draw The Resonance Structure For The Peptide Bond Chegg Com
Lets draw the structure of the die peptide glycerine and serene.
. Meanwhile the basic groups terminal amino group and R group of. Backbone R3 C O H H H N C C R2 R1 O C O H H 118 120 122 121 123 123Å 152Å 145Å 133Å 116 N N C C H C peptide plane peptide plane R1. You can modify the molecule by moving adding deleting or changing bonds or charges.
Structure R O N H R R O N H R _. Peptide bond definition. Peptide bonds are amide bonds between the α-carboxyl group of one amino acid and the α-amino group of another Fig.
Draw the resonance structure of a peptide bond and explain why there is no rotation around the C-N bond The intermediate resonance structure imparts a partial double bond characteristic to the C-N bond thereby prohibiting rotation. You can modify the molecule by moving adding deleting or changing bonds or charges. HN-CHC_NCH-COO- Сн н C - N - C 900.
The atoms C H N O of the peptide bond lies in the same plane like the hydrogen atom of the amide group and the oxygen atom of the carboxyl group are trans to each other. Thus the peptide unit is a planar rigid structure and rotation in the peptide backbone is restricted to the bonds involving the a carbon. The dashed lines indicate the resonance of the peptide bond.
Therefore a reasonable resonance structure can be draw with a double bond linking the carbon and nitrogen and which result in a negative charge on the oxygen and a positive charge on the nitrogen. You do not need to draw nonbonding electrons. The peptide bond would be right here.
It may be an essentially random process. A peptide bond is a special type of amide bond formed between two molecules where an α-carboxyl group of one molecule reacts with the α-amino group of another molecule releasing a water molecule. We discussed three secondary structure motifs.
KHNP is also known as lysylhistidylasparagylproline where the lysine residue is the N-terminal and the proline residue is the C terminal. The peptide bond is also referred to as the isopeptide bond where the amide bond forms between the carboxyl group of one amino acid. So our die peptide will be Oh double Bond.
Cis-trans isomerism Having partial double bond character the peptide bond is planar. Modify the molecule to show the resonance form. At first glance it would seem logical to say that it is sp 3-hybridized because like the nitrogen in an amine the Lewis structure shows three single bonds and a lone pairThe picture looks quite different though if we consider another resonance contributor in which the.
Learn this topic by watching Proteins and Amino Acids Concept Videos. The four atoms that are part of the peptide bond are shown as larger spacefilling models. Draw the resonance structure for the peptide bond below.
The resonance structure prevents rotation around the peptide bond. The real structure of course is a weighted hybrid of these two structures. The resonance structures that can be drawn for the peptide bond indicate that the peptide bond Options is.
Below draw the resonance structure of the peptide bond. 4 pts See page 74 of the textbook. Draw the resonance structure of a peptide bond and explain why there is no rotation around the CN bond.
Which of the following statements concerning the process of spontaneous folding of proteins is false. The result is a planar structure that is stabilized by resonance between the α-carboxyl and α. Which of the following statements concerning the.
You do not need to draw nonbonding electrons. What is the hybridization state of the nitrogen atom in an amide. Amino acids and proteins.
Peptide bonds are formed when the amine group of one amino acid binds with the carbonyl carbon of another amino acid. Resonance and peptide bonds. The intermediate resonance structure imparts a partial double bond characteristic to the CN bond thereby prohibiting rotation.
The C-N distance in a peptide bond is typically 132 Å which is intermediate between the values expected for a C-N single bond 149 Å and a CN double bond 127 Å. Oh and H the bond Oh eight and H two. Created by Tracy Kim Kovach.
The tetrapeptide below is called alanylaspartylglycylleucine. Draw the resonance structure for the peptide bond below. The resonance structure is a significant factor in depicting the true electron distribution.
The resonance structure prevents rotation around the peptide bond. Also shown are the individual dipole moments arrows associated with each bond. Each peptide bond is shown in a shaded box.
24 is asking us to draw the resident structures of the peptide bond so we can start by trying are a peptide bond. Amino acids and proteins questions. The structure at the right shows a peptide bond between the amino acids valine Val and serine Ser.
Draw the resonance structure for the peptide bond below. The peptide bond has approximately 40 double-bond character. The structure of the peptide KHNP at pH 7 is shown below.
We will learn more about peptide bonds and how the cleaving process occurs. The amide structure has two resonance contributors. Draw the resonance structure for the peptide bond shown in the image.
Because the bond between the carbonyl carbon and the nitrogen has a partial double bond character rotation around this bond is restricted. Modify the molecule to show the resonance form. Central dogma of molecular biology.
Charges result in the peptide bond having a permanent dipole. For steric reasons the trans. Therefore a reasonable resonance structure can be draw with a double bond linking the carbon and nitrogen and which result in a negative charge on the oxygen and a positive charge on the nitrogen.
As a result it is rigid. The amino acids are taken from the crystal structure of hemoglobin αVal 132 and αSer 133. Resonance of the Peptide Unit.
And then to get the residents structure we can move the electrons from this double bond up to the oxygen that we can move this lone pair from nation between the carbon and nitrogen. This is a Most important question of gk exam. Has partial double bond character 2.
At pH 7 all of the acidic groups terminal carboxyl group and the R group of histidine are deprotonated. Do not draw nonbonding electrons. The coplanarity of the peptide bond denotes the resonance or partial sharing of two pairs of electrons between the amide nitrogen and carboxyl oxygen.
So theres air Die peptide and were asked to circle the peptide bond. Incorrect It may involve a gradually decreasing range of conformational species. Both and 5.
The real structure of course is a weighted hybrid of these two structures. True Answer Correct It may be defective in some human diseases. Is stronger than an ordinary single bond 3is still not completely understood 4.
Click on the structure below to switch the resonance forms of the peptide bond. Pauling and Corey showed that in small peptides six atoms associated with the peptide bond all lie in a plane.
Solved Draw The Resonance Structure For The Peptide Bond Chegg Com

Oneclass Draw The Resonance Structure For The Peptide Bond Below Modify The Molecule To Show The Re

Peptide Bond Definition Structure Mechanism And Examples

Solved Draw The Resonance Structure For The Peptide Bond Chegg Com

Solved Draw The Resonance Structure For The Peptide Bond Chegg Com
Solved Draw The Resonance Structure For The Peptide Bond Chegg Com
0 comments
Post a Comment